By the beginning of 1915, four months after Britain declared war on Germany, the conflict in Europe was at a standstill. Thousands of miles of trenches crisscrossed Belgium and northern France, and anyone poking their head above ground was liable to attract a sniper’s attention. Desperate to break the stalemate, a young Winston Churchill pushed a plan to attack the Central powers from behind, by invading Turkey. If successful, the opening would allow the Allies to deliver much-needed supplies to the Russians on the Eastern Front and potentially swing the war in the Allies’ favor.
Allied generals believed Gallipoli, a dry peninsula jutting out into the Aegean Sea, was the key to establishing a foothold in Turkey. But the Allied planners underestimated the resistance they would face and didn’t plan for the near-impassable geography: after six months of naval and land assaults the British, French, Australian, and New Zealand soldiers fighting in Turkey had sustained many casualties but taken little land. In August, Allied commanders hatched a scheme to capture a ridge in the center of the peninsula to gain the advantage of higher ground. Among those deployed was a unit of inexperienced Brits sent on a night march through treacherous, boulder-filled gullies.
The soldiers never reached their destination. While stumbling through the unfamiliar terrain they came under attack from 30,000 Ottoman troops supported by machine guns. It was a slaughter. Among the British dead was a promising young physicist who had reshaped the periodic table just before shipping off to war, and his death would reshape the way militaries determined who was and was not sent to war.

Patriotism and social expectations drove Henry Moseley to volunteer for the war, as they had for many of his upper-class peers. Later dubbed the “Lost Generation,” these officers died at almost twice the rate of other British soldiers. Of those boys who attended the same prestigious boarding school as Moseley, more than 20% were killed. Many were likely well on their way to becoming accomplished philosophers, scientists, and mathematicians.
Moseley, however, was one of the few who had already demonstrated his potential. He came from a long line of scientifically inclined men, most of them also named Henry Moseley. His great grandfather, a Calvinist preacher turned unlicensed physician, hung out with the likes of Erasmus Darwin and Thomas Beddoes, members of a radical group of writers and intellectuals whose research contributed to new theories of medicine and politics. His mathematician grandfather presented a paper to the Royal Society with new standards for naval engineering that were adopted by shipbuilders across Europe. His father, a naturalist and anatomy professor at the University of Oxford, sailed on the famous HMS Challenger, a warship turned scientific-research vessel.
In 1891, when Moseley was only four years old, his father succumbed to a years-long illness and left behind a fortune that allowed young Henry, his mother, and his two older sisters to live comfortably, tended by servants in their large home in Dorset in southwestern England. The older of Moseley’s sisters died in her teenage years; the younger, Margery, taught him to identify plants, birds, and other wildlife and inspired him to pursue the family’s scientific traditions.

Moseley developed a love for experimentation at Eton College, a private boarding school in Windsor. At the school’s annual Scientific Society Exhibition—essentially a high-stakes science fair—he demonstrated how a bubble of phosphine gas exploding on the surface of water emits a flash of light. “The smell is of course appalling,” he wrote with apparent delight. (The gas is also highly toxic.)
As an undergraduate at Trinity College, Oxford, Moseley fit right in with the other students. He attended church, rowed on the Thames, and joined the debate club, sometimes to the detriment of his academic pursuits. One of his tutors described him as “very erratic & rather untidy but works very hard.” When a student newspaper called for recruits for a new Officers Training Corps, Moseley enlisted. Writing to Margery, he told her he could “find no sound argument with which to confute the advocate of universal service.”
When he wasn’t rowing, drilling, or studying Plato, Moseley was reading scientific journals and devising experiments. He built an early version of a cloud chamber, which shows the paths subatomic particles take through water vapor, based on the designs of physicist Charles Thomson Rees Wilson. Moseley also wrote class papers on the structure of the elements and the radiation they emitted. By the time he graduated in 1910, he already had an offer to join Ernest Rutherford, a renowned researcher on radioactivity, at the University of Manchester.
Discoveries in chemistry and physics were being made at an unprecedented rate in the decades before World War I. The noble gases, the group of inert elements that includes neon, argon, krypton, xenon, and radon, were identified only in the late 1890s. (The first noble gas discovered, helium, had been detected earlier through observations of the sun.) German physicist Wilhelm Röntgen discovered X-rays in 1897, and in 1902 Rutherford, along with his colleague Frederick Soddy, made real the dreams of alchemists when he found that elements could transform into other elements through radioactive decay. So when Moseley accepted Rutherford’s offer to join him at Manchester, the budding scientist was poised to uncover something novel about the nature of matter.
But things did not go so well in Manchester. Rutherford tasked Moseley with studying radioactive isotopes and teaching undergraduate courses, assignments Moseley found dreary. Rutherford was unhappy when Moseley shifted his studies to X-rays, and in November 1913 Moseley made the surprising decision to leave Manchester and return to Oxford without the promise of a job. As he waited for a position to open up, a sympathetic colleague offered him laboratory space in which to continue his X-ray research.

Around this time Moseley read a paper written by Antonius van den Broek, an amateur Dutch physicist. Van den Broek believed that the elements in the periodic table should be ordered based on their atomic numbers rather than by atomic weight, the latter method having been adopted as the standard practice since the first modern periodic systems appeared 50 years earlier. Tables built on atomic weights were imperfect—they contained irregular gaps and ambiguous sequences—but no one had yet created a superior alternative. Van den Broek made the bold claim that the seemingly superficial atomic number assigned to each element was instead an inherent physical property of that element. That property, he speculated, might be its positive charge. In his makeshift laboratory at Oxford, Moseley realized that an X-ray spectrometer he had recently built, based on a design by the father-and-son team William and Lawrence Bragg, could put van den Broek’s theory to the test.
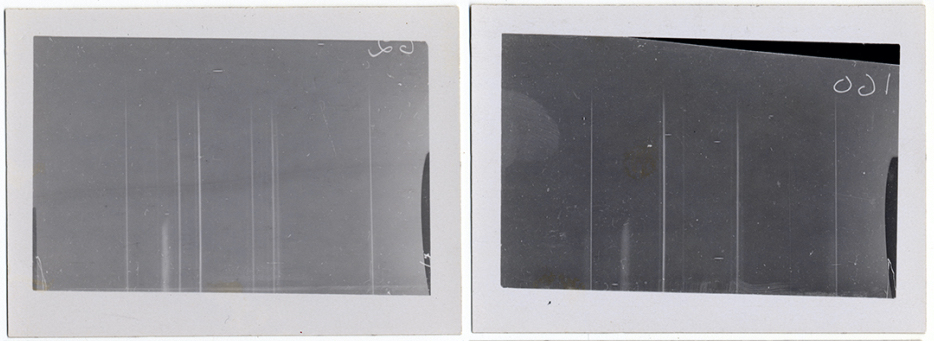
Moseley used his device to direct a stream of X-rays at a sample element, which responded by expelling energy in the form of its own X-rays, energy that left telltale streaks on photosensitive paper. Each element, it turned out, left streaks at a unique angle, allowing Moseley to calculate the frequency of that element’s X-rays. Testing every element he could get his hands on, from hydrogen to gold, Moseley showed that X-ray frequency increases as atomic numbers grow larger. Van den Broek was right. (Later, atomic numbers were found to equal the number of protons in the nucleus of each element.)
Moseley presented his results in two papers published between 1913 and 1914; chemists and physicists around the world immediately took notice. Moseley’s research didn’t just force a reorganization of the periodic table: it also had practical applications, including predicting as-yet-undiscovered elements through their atomic numbers. For the first time in history scientists had a clear map directing them to brand-new elements (along with near-certain scientific renown), and the race to find them was on.
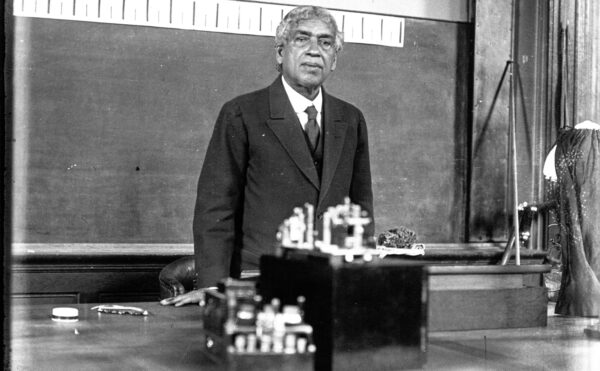
French chemist Georges Urbain entered the race a bit too early with “celtium,” a substance he believed to be a contender for atomic number 72. He sent samples of the stuff to Moseley, who ran it through his spectrometer and found it was simply a mixture of two known elements. (Urbain, though, refused to accept the results.)
The ease with which Moseley debunked celtium helped him recognize a second use of X-ray spectroscopy: he could swiftly identify the elemental makeup of just about any substance. For instance, the rare earth metals, a group of chemically similar elements, took years to analyze because their appearance and behavior made them nearly indistinguishable. Moseley could now measure their frequencies and differentiate europium from lanthanum, or another rare earth, in mere minutes.
In the spring of 1914 Moseley was invited to share his research at the annual meeting of the British Association for the Advancement of Science, held that year in Australia. When war broke out in the summer, Moseley cut the trip short and rushed back to England, where he volunteered as a signaling officer, responsible for sending communications in Morse code and semaphore. After training at a military base in the town of Aldershot, he shipped out with his unit to Gallipoli on June 13, 1915.
By the beginning of July, Moseley was camping under thistle-covered Turkish cliffs with his fellow soldiers, far removed from his upper-class comforts in England. Moseley’s life had become a blur of flies and sand, which he bemoaned in letters to his mother amid gripes about his fellow soldiers, whom he accused of stealing his shaving razors. Like most of the soldiers, he suffered from bouts of dysentery. Finally, after weeks of lingering in the dry scrub, British generals ordered the ill-fated attack.

Moseley was shot in the head, purportedly by a sniper, as his unit was overrun. Five months later the Allies retreated from Gallipoli. At least 140,000 Allied troops were killed or wounded during the campaign, with nothing to show for it. The war continued in a stalemate for another two years.
Moseley was only 27 years old when he died. He had reformed the periodic table and invented a new type of X-ray spectroscopy in less than four years of postgraduate work. What else might he have achieved if he had lived? Scientists around the world were shocked by the absurdity of the situation: a brilliant mind like Moseley’s should not have been anywhere near the front lines.
Rutherford wrote, “The loss of this young man on the battlefield . . . [is] a striking example of the misuse of scientific talent.” Science-fiction author and biochemistry professor Isaac Asimov later asserted that “in view of what he might still have accomplished . . . his death might well have been the most costly single death of the War to mankind generally.”

Rutherford and other British scientists successfully petitioned the British War Office to revise its recruitment policies: never again was a promising scientist knowingly assigned to a combat role. Instead, they were ordered to build wartime technology. Scientists may have been spared from firing weapons on the battlefield, but they still were often charged with designing them in laboratories.
If he had survived, Moseley might have been a contender for the 1916 Nobel Prize in Physics or Chemistry. The prizes for those subjects were not awarded that year. Charles Barkla, another physicist who had published research on how X-rays were absorbed and emitted by elements in unique patterns, received the award for physics in 1917. Of course, it was Moseley who figured out how to measure and apply those patterns.
Some of the missing elements Moseley predicted in his updated periodic table were so rare and unstable they were not discovered until decades later, when scientists learned how to artificially produce them. The last of those elements, promethium, was found in the by-products of a nuclear reactor in 1945.