Florence Ogilvy Bell
What do shark fins, wool, and DNA have in common? Physicist Florence Bell studied them all with X-ray crystallography.

Physicist Florence Bell (1913–2000) once remarked that her greatest achievement was being the first woman in the Royal Air Force to have worn trousers. This claim was far too modest, for it was Bell who showed in 1938 that X-rays could be used to reveal the regular, ordered structure of deoxyribonucleic acid (DNA), the genetic blueprint of all living organisms.
This work had a surprising origin: it came about through research she was doing on the structure of wool fibers for the textile industries of Northern England with William Astbury, a physicist and molecular biologist. But it nevertheless laid important foundations for the later X-ray studies of DNA made first by Maurice Wilkins and Rosalind Franklin at King’s College in London. Their work proved vital to identifying the double-helical structure of DNA in 1953.
X-ray Visions
Bell grew up in London and attended what is known today as Haberdashers’ Girls’ School in Acton, where she excelled both in the classroom and on the sports field. In 1932 she was accepted to a program of study in the natural sciences at Girton College, University of Cambridge, which was dedicated to training women in science and engineering; it was also pioneering in its support of women’s cricket. After completing her studies, Bell began working at the Cavendish Laboratory in Cambridge. Here, she was introduced to a powerful scientific tool known as X-ray crystallography, which uses the scattering of X-rays to determine the spatial arrangement of atoms and molecules in crystals.
For a young scientist like Bell, this technique offered an ideal opportunity to blaze a new trail in an exciting field of science. X-ray crystallography had been developed in 1913 by physicist William Bragg and his son, Lawrence. They were working at the University of Leeds, where they had used the technique to visualize the molecular arrangement of crystalline substances (e.g. diamonds and table salt). But by 1936 when Bell joined his laboratory in Manchester, Lawrence Bragg was applying this method to more complex and biologically active compounds such as sterols.
We know that Bell quickly developed a talent and expertise in this field because of the way Lawrence Bragg responded to a letter he received in 1937. The letter came from William Astbury, who had also developed an interest in applying X-ray crystallography to biological compounds. Astbury’s work had begun with studies of wool, an essential raw material used in the textile industries for which Leeds had been a center since the Middle Ages.
From his X-ray studies of wool, Astbury made key discoveries about the molecular nature of proteins and became internationally renowned for his expertise in the X-ray study of biological fibers, including hair, nails, and feathers. As his success grew, so too did his research program and his need for another X-ray crystallographer. Though Bragg was reluctant to see Florence Bell go, he had no hesitation in recommending her, describing her as “somebody who is capable and experienced and really gets on with the job.”
Wool Weaves a Path to the Double Helix
One of the jobs that Bell got “on with” during her time in Astbury’s laboratory was her PhD thesis. With chapters on jellyfish, shark fins, and uncommon hair diseases, her doctoral thesis might seem haphazard. But a common theme united this eclectic range of subjects. In each case, Bell used X-rays to study the shape of the protein fibers that made up her materials of study.
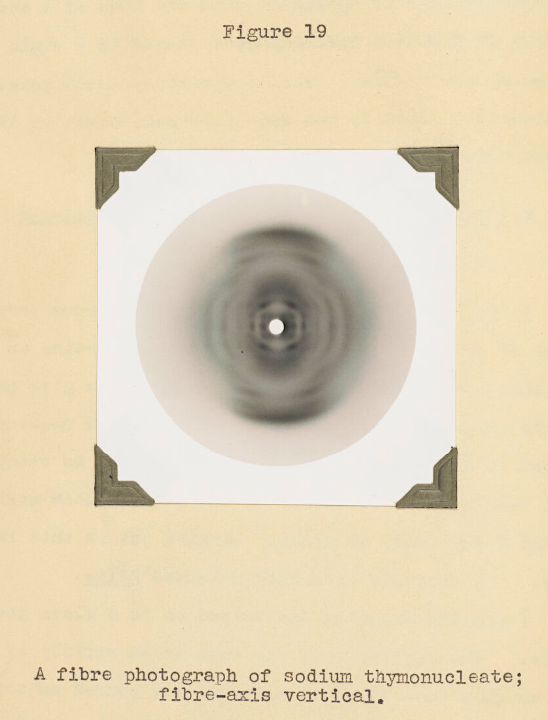
One chapter of Bell’s thesis in particular stands out for its significance in the history of the life sciences. It describes structural studies of a unique kind of biological fiber. This substance, known at the time as “thymonucleic acid,” was chemically distinct from proteins like the keratin in wool because of its high phosphorus content. Today we know this substance as DNA. Taking X-ray images of this acid was not pleasant: exposure times could last more than 10 hours, and the work required a lot of stumbling around in a dark room with red-hot X-ray tubes operating at dangerously high voltages.
But Bell was tenacious and skilled. In 1938 she and Astbury proposed an early model for the structure of this substance based on her X-ray images. In their model, the molecule was composed of only a single chain of sugar and phosphorus, not two chains entwined around each other into a double helix as we think of the structure today.
Nevertheless, Bell and Astbury showed that the four chemicals called the “bases,” which we now know carry the genetic code, were stacked adjacent to each other, like “a pile of plates.” They were able to determine that the distance between adjacent bases was about 0.34 nanometers, a measurement that gave James Watson and Francis Crick an important foothold when they began their own work on DNA structure 13 years later.
LEARN MORE
What is DNA made of?
The DNA molecule can be thought of as a twisted ladder. The outermost parts are made up of alternating units of deoxyribose sugar and phosphate chemically bonded to each other into a chain. The rungs of the ladder consist of four cyclic, nitrogen-containing compounds known as “bases.” There are four different types of bases found in DNA: adenine, cytosine, guanine and thymine, which are abbreviated as A, C, G, and T, respectively. Depending on their ring structure, each of these bases belongs to one of two groups known as purines or pyrimidines.
One of the key insights that emerged from the double-helical model of the DNA molecule was that, thanks to intermolecular forces of attraction known as hydrogen bonds, adenine on one chain always pairs up with thymine on the other chain, while guanine pairs with cytosine. This specific pairing not only holds the two chains of the helix together but, as Watson and Crick realized, it offers a means by which DNA is able to copy itself.
Ultimately, Bell’s work showed that the regular, ordered structure of this substance could be revealed by X-ray methods, paving the way for the identification of the double-helical model.
“Woman Scientist Explains”

Astbury had immense respect for Bell’s intellect and expertise. He nicknamed her his vox diabolica or “devil’s advocate” for her willingness to challenge him when he drew conclusions ahead of his data. In 1939, when the Institute of Physics held a conference in Leeds, Astbury asked Bell to present on the work of their laboratory. Local newspaper headlines, however, focused less on the scientific content of Bell’s talk than on her gender.
Reporting on Bell’s presentation with the headline “Woman Scientist Explains,” the local press seemed stunned not only that a woman could do science, but that she might also be able to talk eloquently about it. Sadly, Astbury himself was not exempt from such views. Around this time, he confessed to being “one of those people that still maintain that there is a creative spark in the male that is absent from women, even though the latter do so often do such marvelously conscientious and thorough work…”
Despite this general outlook, when Bell was called up for military service in 1941, Astbury pleaded with the War Office, hoping she would be allowed to stay in his lab. At his request, the University of Leeds even held her position open. But it was not to be, and later that year Bell left Astbury’s lab to join the Women’s Auxiliary Air Force where, according to one of her sons, she worked on the development of radar. There she met Captain James Sawyer, a U.S. serviceman. The couple married in December 1942 before moving the following year to begin a new life in the United States.
After her move to the U.S., Bell worked for a time as an industrial chemist with the Magnolia Petroleum Company in Beaumont, Texas. She eventually left this career to look after her four adopted children and died at the age of 87 on a visit to the U.K. Her death certificate recorded her occupation as “housewife,” a reflection of the radical change her life had undergone in Texas. But this label gives little indication of her earlier achievements. With her X-ray studies of thymonucleic acid, Bell had proven Astbury’s view of women in science wrong.
LEARN MORE
What if…Florence Bell had stayed in Leeds?
It is interesting to speculate on how events might have unfolded had Astbury’s pleas to the War Office been successful. Although Bell’s departure was a major blow, his work on DNA did not come entirely to a halt. In 1951 Astbury’s research assistant, Elwyn Beighton, took some new X-ray photos of DNA that are identical to the now famous Photo 51 taken a year later by Rosalind Franklin and Raymond Gosling. Photo 51 provided an important clue to solving the structure of DNA. Astbury, however, did nothing with Beighton’s photograph; he just filed it away.
Had Florence Bell still been on the scene, it is difficult to believe that she would have allowed him to do this. And while it may be a stretch to suggest that this photograph alone would have allowed her and Astbury to identify the structure of DNA, she might certainly have encouraged Astbury to show it to his friend, U.S. chemist Linus Pauling, when they visited in 1952. As Pauling’s own efforts to solve the structure of DNA had been hamstrung by a lack of good quality X-ray images, the photo from Astbury’s lab might have altered the course of history.
But she had also proven Astbury to be right when he described science as “the most wonderful thing that I know…not just bombs, luxuries, wireless, and what-not. It is man’s greatest intellectual adventure, his journey into the unknown.” When Bell first arrived in Leeds, no one could have predicted that industrial research on threads of wool would provide the first glimpse into the structure of a very different kind of thread: her work ultimately paved the way for contemporary understanding of the biological fibers through which organisms carry their genetic information. Hers was truly a journey into the unknown.
Glossary of Terms
Sterols
Sterols are lipids (fats). They are a subgroup of steroids and are essential to the normal functioning of plants, animals, and fungi.
Back to top
Middle Ages
The period in European history between the fall of the Western Roman Empire (5th century CE) to the beginning of the Renaissance (13th-15th century CE). As these date ranges suggest, periodization is often debated by historians.
Back to top
Nanometer
One nanometer is one billionth of a meter. A strand of human hair is about 80,000–100,000 nanometers in thickness.
Back to top
Further Reading
Astbury, William T. and Florence Bell. “X-Ray Study of Thymonucleic Acid,” Nature 141 (1938): 747–748.
Hall, Kersten. The Man in the Monkeynut Coat: William Astbury and the Forgotten Story of How Wool Wove a Road to the Double-Helix. Oxford University Press, 2014/2022.
The Naked Scientists. “Florence Bell: An Unsung Heroine of DNA.” BBC Podcast, June 17, 2022.
“Wall of Inspiration Reflects Science’s Known and Unsung Heroes.” HHMI’s Janelia Research Campus, April 23, 2023.
Support
Support for this biography was made possible by the Wyncote Foundation.
You might also like

SCIENTIFIC BIOGRAPHIES
Linus Pauling
A Nobel laureate and prolific researcher who made significant contributions to our understanding of chemical bonding and structure.

DISTILLATIONS MAGAZINE
It’s Nothing New: Sexism in the Lab
Why the recent findings of the National Academies of Science, Engineering, and Medicine are enlightening, even if they aren’t surprising.

DISTILLATIONS MAGAZINE
Hennig Brandt and the Discovery of Phosphorus
An engraving hints at the ways art and science were intertwined in the Age of Enlightenment.



