William Ramsay
Winner of the 1904 Nobel Prize in Chemistry, Ramsay helped establish the noble gases as a new group in the periodic table. He first discovered argon and then helium, followed by the other noble gases.

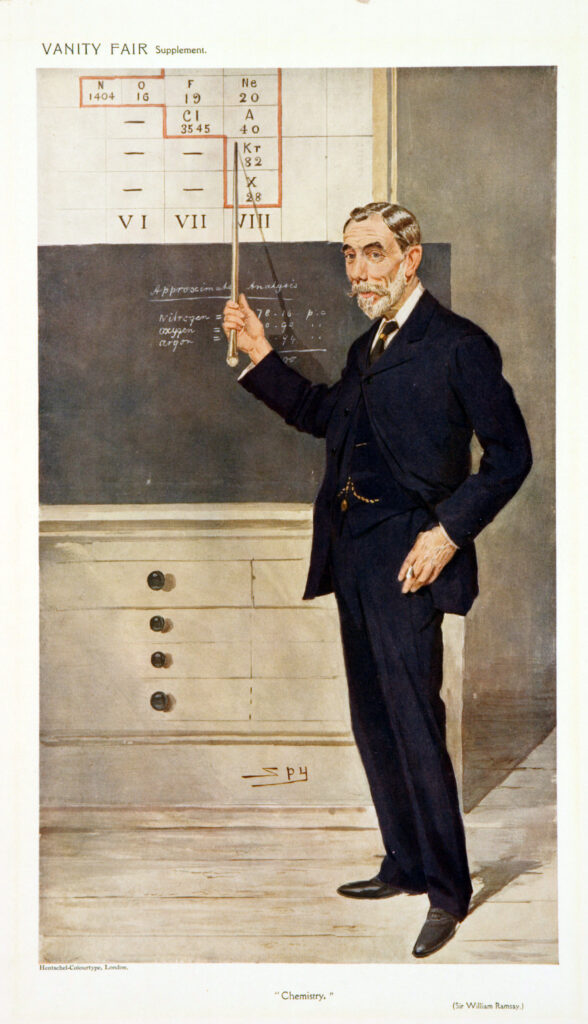
The Scottish chemist William Ramsay (1852–1916) is known for work that introduced a whole new group to the periodic table, variously called over time the inert, rare, or noble gases.
In the last decade of the 19th century he and the famous physicist Lord Rayleigh (John William Strutt, 1842–1919)—already known for his work on sound, light, and other electromagnetic radiation—carried out separate investigations, for which they received Nobel Prizes in 1904, Ramsay in chemistry and Lord Rayleigh in physics.
Inventive Researcher
Ramsay began his studies in his native city of Glasgow and completed a doctorate in chemistry at Tübingen, focusing on organic chemistry. On his return to Great Britain and his appointment to academic posts at the University of Bristol and then at University College London, he became known for the inventiveness and scrupulousness of his experimental techniques, especially for his methods for determining the molecular weights of substances in the liquid state.
Discovery of Noble Gases
In 1892 Ramsay’s curiosity was piqued by Lord Rayleigh’s observation that the density of nitrogen extracted from the air was always greater than nitrogen released from various chemical compounds. Ramsay then set about looking for an unknown gas in air of greater density, which—when he found it—he named argon.
While investigating for the presence of argon in a uranium-bearing mineral, he instead discovered helium, which since 1868 had been known to exist, but only in the sun. This second discovery led him to suggest the existence of a new group of elements in the periodic table. He and his coworkers quickly isolated neon, krypton, and xenon from the earth’s atmosphere.
Uses of Noble Gases
The remarkable inertness of these elements resulted in their use for special purposes, for example, helium instead of highly flammable hydrogen for lighter-than-air craft and argon to conserve the filaments in light bulbs. Their inertness also contributed to the “octet rule” in the theory of chemical bonding (see Gilbert Newton Lewis). But in 1933 Linus Pauling suggested that compounds of the noble gases should be possible. In 1962 Neil Bartlett, working at the University of British Columbia and later at Princeton University, prepared the first noble gas compound—xenon hexafluoroplatinate, XePtF6. Compounds of most of the noble gases have now been found.



