With the world in the grip of the COVID-19 pandemic, researchers are working with unprecedented speed and levels of collaboration to develop a vaccine for the disease. While we await the fruits of that labor, other medical scientists are working on a serum therapy they hope can help reduce the severity of the illness and hasten the recovery of COVID-19 patients.
Vaccines stimulate the body to produce its own immunity to a specific disease, but serum therapy takes disease-fighting chemicals (antibodies) from the blood of recovered patients and transfers them to the sick to boost their defenses. For the therapy to work for COVID-19 patients, researchers must show that those who recover from the disease gain a lasting resistance to the virus that causes it. Such resistance to reinfection commonly occurs after many viral and bacterial illnesses, but it’s yet to be confirmed whether this holds true for the new coronavirus. If confirmed, this approach—often referred to as convalescent plasma therapy—could save lives and reduce the number of new infections during the year or more needed to test and roll out a new vaccine.
While the methods being used to create a serum therapy for COVID-19 are modern, the technique itself is old, pioneered more than a century ago in the fight against diphtheria. The same approach later provided hope and sometimes relief for pneumonia and polio patients, until it was displaced for all three diseases by vaccines.

When the first Nobel Prizes were awarded in 1901, German physiologist Emil von Behring received one for his work both in showing serum therapy could be used as a general technique to fight infectious diseases and in being the first to use it on a patient. The Nobel committee observed that he had “opened a new road in the domain of medical science and thereby placed in the hands of the physician a victorious weapon against illness and deaths.”
Von Behring was among a group of doctors who in the early 1890s pioneered serum therapy to combat the heart-breaking scourge of diphtheria, which killed up to half the children sickened by it. Injected serum reduced the mortality rate to 15%, sometimes working like a medical miracle: children who had been gasping for breath and were near death would be breathing normally within a few hours. Newspapers in Europe and the United States trumpeted the success of this antitoxin, generating widespread public enthusiasm for a novel lab creation.
Concepts we take for granted, such as antigens and antibodies, did not yet exist. Von Behring and his French competitors, Émile Roux and Alexandre Yersin, knew only that some chemical found in the blood of recovered patients acted against the bacterium that causes diphtheria. This antitoxin remained after blood cells were removed, leaving the blood serum behind; when this serum was injected into an infected patient, it strengthened the body’s ability to fight the disease.
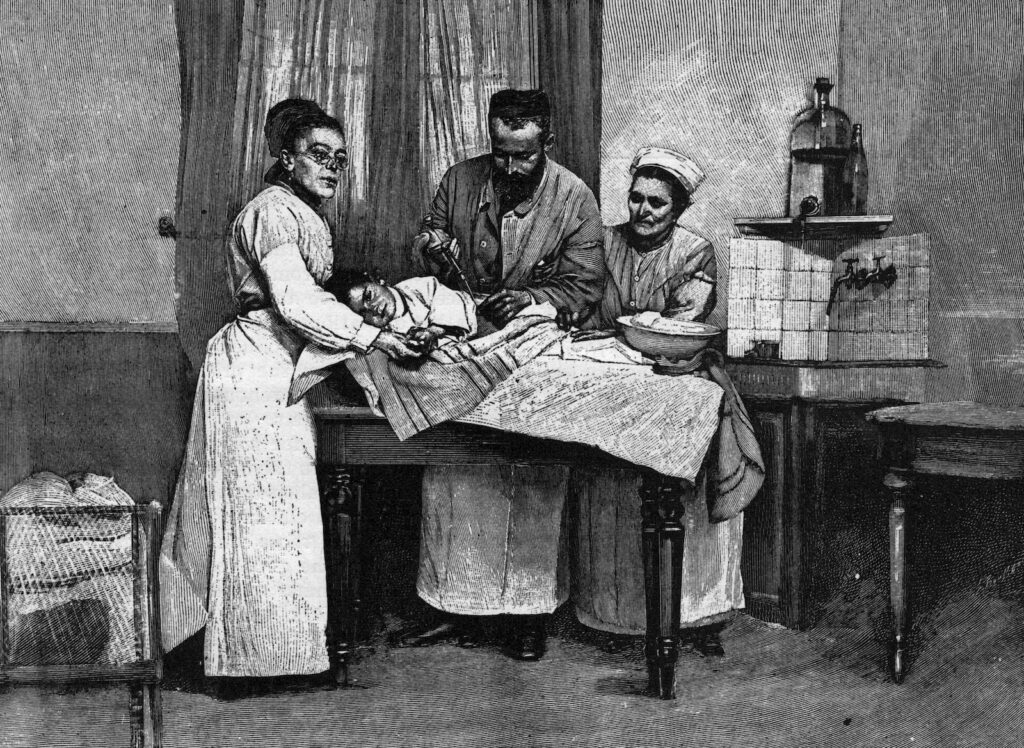
Scientists at the Pasteur Institute in Paris quickly scaled up their blood-collecting techniques, realizing that the scant pint of serum they could get from a single patient’s blood wouldn’t add up quickly enough. So they began injecting docile horses with increasing doses of diphtheria toxin, effectively immunizing them and turning them into blood serum factories with a single product: immunity.
The diphtheria breakthrough and the introduction of serum therapy for tetanus opened up a whole new world of biological remedies, products created in the laboratory by living creatures, distinct from the old world of chemical and botanical drugs. Serum therapy for pneumonia was a revelation when it appeared in the 1930s, and it saved thousands of lives before being replaced by penicillin—another biological product—in the 1940s. In time vaccines for diphtheria and pneumonia displaced the need for the more cumbersome serum therapy.

In the early 1950s Americans faced summer after summer of terrifying polio epidemics. Lacking a vaccine and with the public clamoring for treatments, some doctors turned to serum therapy, hoping to reduce the number and severity of cases. In a 1951 test involving 6,000 children in Utah, half received gamma globulin, an infection-fighting protein in blood serum; the rest were given a placebo.

While the numbers reported by the press seemed favorable, the difference in outcomes between the two groups was not statistically significant. Nonetheless, with the public growing more and more desperate, the unproven treatment was applied on a larger scale. In 1953 and 1954 more than 200,000 American children received gamma globulin in an attempt to prevent another polio outbreak.
Despite the limitations of gamma globulin—it was expensive to produce and was thought to provide only brief protection from polio—as well as problems with the administration of the testing program, the trial nevertheless set the stage for large-scale polio vaccine testing. American parents were clearly willing to enroll their children in a promising trial even with no guarantee of safety or efficacy.

In the summer of 1954 Jonas Salk’s polio vaccine was finally ready for testing. Nearly two million children were separated into three groups: vaccine recipients, placebo recipients, and untreated controls. The test used a double-blind approach: neither the recipients nor the scientists running the experiment knew who had received a placebo and who had been given a vaccine. Parents and doctors anxiously awaited the enormous field trial’s results, which were due in April 1955. Twenty-seven million doses of vaccine were stockpiled, ready to be rushed to the first cohort of nine million children if the vaccine proved effective.
On April 12 news of the vaccine’s spectacular success was announced, a new medical miracle heralded around the world. Mass injections began immediately across the United States, and the use of blood serum as a treatment for polio ended.
Trials of a blood serum therapy for COVID-19 began in late March. With new trials coming online at hospitals around the country, serum treatments could act as a bridge between life without a vaccine and life with a vaccine.
If the pattern of history holds, the very real if limited benefits provided by the serum proteins of recovered patients will be replaced by the active immunity of a vaccine. Yet no matter how strongly we might wish for the magic bullet of an immediate vaccine, history reminds us that a 19th-century therapy might still prove itself a vital part of our 21st-century armamentarium.




