Zurich, 1957. Freudianism is enjoying a revival, thriving amid the anxiety of two nuclear superpowers racing to build bigger missiles. Famous psychiatrists have come to Switzerland for the Second International Congress of Psychiatry. Again and again they talk about the way many mental illnesses leave no anatomical signs, evidence that these diseases must be psychological rather than physical in nature.
Listening closely, remembering everything, is Wayne Woolley. He’s a biochemist, and he’s been experimenting with LSD. He’s not taking it himself to chase altered states in the way Timothy Leary will soon use the drug or the way Aldous Huxley had experimented with mescaline a few years earlier. No, Woolley only gives drugs to mice. But Woolley also knows LSD has a very similar chemical structure to serotonin, a chemical that’s recently been discovered in the brain. The structures are so similar, in fact, that LSD appears to be able to masquerade as serotonin on a molecular level and disrupt brain function in intriguing ways.

It’s the similarity between LSD and serotonin that makes Woolley disagree with the Freudians. To him the lack of anatomical signs suggests many mental illnesses might be caused by biochemical imbalances. By the time he arrives in Zurich, Woolley is developing what he calls the serotonin hypothesis. It’s an argument that a variety of mental illnesses result from “changes in the functioning amounts of a few specific chemical substances in the brain.”
During Woolley’s lifetime, researchers have discovered how the right amount of specific chemicals could make sick bodies well again. Wayne Woolley has lived these discoveries both as a patient and as a researcher. Maybe, he thinks, similar chemical fixes could work for minds.
Woolley’s own mind astonishes his colleagues with feats of memory and insight. But it is part of a body being slowly destroyed by diabetes. He works with fierce intensity, spending days and nights in the lab.
He’s certain that understanding the biochemistry of the brain can help people with mental illness just as the discovery of insulin had helped diabetics. But would he have time to find out?
A Cold Place
Dilworth Wayne Woolley was born in 1914 in Raymond, Alberta, a small, wind-swept prairie town 80 miles northeast of Glacier National Park. His parents had migrated from Utah, one of many Latter-day Saint families in Raymond, which had been founded at the start of the 20th century by the Mormon mining magnate Jesse Knight, who hoped to develop a sugar beet industry. Wayne’s father, Andrew Dilworth “A. D.” Woolley, farmed his own land, while Wayne’s mother, Henrietta Christine Woolley (nee Schoenfield), kept house and took care of his older brother, Glenn, and older sister, Arla.

If Wayne Woolley had been born just a few years earlier, he would never have survived childhood. Wayne was born with juvenile diabetes, a death sentence at the turn of the 20th century. Diabetic children lived only a few agonized years, wasted by their bodies’ inability to metabolize sugar. But in 1921 a team of scientists at the University of Toronto isolated insulin for the first time and soon turned it into a lifesaving treatment. Woolley’s young life was saved when insulin treatment became available in 1923.
Published sources say almost nothing about Woolley’s childhood except to highlight his precociousness and ill health. In addition to diabetes, Woolley also suffered from the nutritional deficiency rickets. He recovered after treatment with cod-liver oil, a potent source of vitamin D. His high school newspaper reported that he received his diploma at age 13, though he waited four more years before starting college. Presumably he worked on the farm during that time. He tinkered and wrote as well, telling a colleague much later that he published his first article at age 17 in a magazine of popular mechanics. When Woolley graduated with first-class honors in chemistry from the University of Alberta in Edmonton in June 1935, “the ice was still on the river. That was a cold place,” he remembered.
Wisconsin
Woolley warmed up by moving south to a hotbed of biochemical research—the University of Wisconsin. He survived on special funds set aside to support graduate students in what was then called the Department of Agricultural Nutrition. Amid the Great Depression, “fifty bucks a month as a research assistant looked pretty good,” Woolley’s fellow graduate student Robert Burris remembered. Graduate work certainly beat digging new sewers with the Work Projects Administration.
Not that anyone would put a shovel in Woolley’s slender hands.
“When he first came to Madison, I thought he was a high school boy,” wrote his department chair, E. B. Hart, in a 1938 recommendation letter. But after seeing him work, Hart declared, “We think very highly of Woolley . . . he has brains to spare.” His fellow students agreed.
“He could retain anything that came through his marvelously equipped mind,” recalled Burris, still amazed five decades later. When Burris had trouble isolating an amino acid, “I’d just come down and talk to Woolley,” he remembered. “Woolley’d say, ‘Well I’ll tell you, if you want to clean that thing up there’s a nice 1912 paper by Krebs on page 242 of Zeitschrift für Physiologische Chemie.’”
What was the source of this powerful focus? Some friends and colleagues speculated that the life-saving treatments Woolley received as a child instilled in him a sense of obligation to science. If so, Woolley began to repay his debt through intense work in the lab and wide-ranging reading of scientific literature. William Harold Peterson oversaw Woolley’s primary research, on growth factors in bacteria. Chemicals that enable or inhibit the growth of bacteria could be valuable for medicine as well as the many agricultural and industrial products made through fermentation. Peterson was a generous mentor, “whose picture Wayne kept on his desk for the rest of his career,” a colleague recalled.
But the research that made Woolley’s reputation came out of an effort to understand pellagra, a devastating nutritional disease. At the time, pellagra caused as many as 7,000 deaths a year, mostly among poor Southerners, who often subsisted on a monotonous diet heavy in corn and dried pork.

Wisconsin professor Conrad Elvehjem had been studying a canine disease, black tongue, which was understood to be a model for pellagra in humans. Elvehjem’s work showed that dogs fed a liver extract quickly got better—but what was the essential nutrient within the extract? A key insight came in 1937 when Elvehjem’s student Robert Madden gave an experimental dog nicotinic acid, then considered a poison. In one version of the story, this inspiration came from Madden, Woolley, and fellow graduate student Esmond Snell’s late-night lab conversation about a recent paper showing nicotinic acid was a growth factor for diphtheria bacillus and Staphylococcus bacteria.
Knowing what to look for, Woolley was able to crystalize and characterize nicotinic acid from the liver extract in a matter of days. These crystals cured the dogs. In 1938, Elvehjem, Woolley, and two other colleagues announced they had isolated and identified nicotinic acid as the anti–black tongue factor (now known as niacin, or vitamin B3). Their paper listed Elvehjem as first author and Woolley last.
Woolley, who had produced virtually all the results reported in the paper, felt slighted. “Woolley did not have Elvehjem’s picture on his desk,” his colleague noted drily.
Ultimately, the slight only damaged Woolley’s pride—discovering the factor that could eradicate pellagra was career-making. Years later, further research by Woolley revealed that the human body cannot make use of the niacin in corn.
Untreated pellagra also led to a form of dementia. Indeed, pellagra-induced psychosis was the major reason for psychiatric hospitalization in the United States during the early 20th century. Once pellagra was understood as a blockage of niacin, this form of mental illness could be cured almost immediately, a fact that Woolley would cite much later as evidence for the serotonin hypothesis.
Love and Blindness
Perhaps it’s no surprise that a man driven to spend night and day in the lab finds love there. Woolley finds Janet McCarter, a bacteriologist who’s an expert on tubercle bacilli and the biological properties of infectious diseases. She’s eight years his senior, the daughter of a school superintendent from Duluth, Minnesota. Cold places, warm hearts.
When they meet, McCarter is an accomplished scientist making a career for herself. The fields of bacteriology and biochemistry are relatively more accepting of women scientists during this period of American history, and McCarter is one of several women with PhDs working in Wisconsin’s “bacti” department. She writes research papers with other women in the department, including Elizabeth Kanne and Dorothy Powelson. Wisconsin promotes her from instructor to assistant professor in 1941. The Guggenheim Foundation awards her a fellowship in 1944, part of an unprecedentedly large class of women among the awardees. That much is well documented.

Scarcely documented at all are Woolley’s and McCarter’s personal lives. We know they meet in Madison, perhaps as early as fall 1935, travel together in 1939, and coauthor a research paper in 1940.
Woolley moves to New York City to work at the Rockefeller Institute for Medical Research in 1938. Almost six years later, McCarter comes to Columbia University to spend her Guggenheim fellowship year studying tuberculo-proteins. They marry on June 24, 1945.
Published sources say nothing more about their relationship during this period. It is possible they (or she) are trying to protect her career by delaying marriage. (It’s an era when women routinely lose their jobs when they marry.) But New York will be where their lives and science are transformed.
The Rockefeller Institute, a world leader in biochemistry research, is a sanctuary of science on the east side of Manhattan. A dining room keeps staff on campus and well fed, seated at eight-person tables chosen to promote deep but focused conversations.
“There never was a symposium,” waxes biologist René Dubos, “more scientifically productive and intellectually pleasurable than those held daily in the lunchroom of the Rockefeller Institute, though coffee and ideas were the only intoxicants.”
It’s the ideal place for Woolley. The institute’s directors, first Simon Flexner and then, after 1935, Herbert Spencer Gasser, seek to maintain tranquility and intellectual ferment by recruiting people “imbued with a spirit of cooperation.” Gasser recruits researchers with broad interests and deep curiosity, people unafraid to move between research directions. He must have been delighted to read a recommendation letter by Harry Steenbock, the man who discovered that vitamin D could cure rickets. Steenbock described Woolley as an omnivorous reader who keeps abreast of many lines of research, “modest” and “a fine chap,” yet the kind of confident person who “translates his knowledge into action with little if any hesitancy.”
But in 1939, shortly after starting at Rockefeller, Woolley takes an uncharacteristic break from the lab.
Insulin may have saved Woolley’s life, but diabetes continues to torment the young scientist. In Madison, during his early 20s, Woolley began experiencing retinal hemorrhages, a complication of the disease that blurred his vision. Surgical efforts to treat the hemorrhages fail. Recognizing that he is losing his eyesight, Woolley and McCarter travel the United States by train, to see as much as possible while he still can. He’ll remember it all.
Back in the lab, Woolley and McCarter publish a paper titled “Antihemorrhagic Compounds as Growth Factors for the Johne’s Bacillus,” in October 1940. It’s a logical bridge between McCarter’s research on tubercle bacilli and Woolley’s studies of bacteria growth factors, but it also reveals a clear interest in vitamin K, which the agricultural biochemist H. J. Almquist had labeled the “anti-hemorrhagic vitamin” a few years earlier. Are they hoping that biochemical research can save his sight the way it had saved his life as a boy?

Woolley leaves behind hundreds of thousands of words about biochemistry but almost none about himself. He refuses to describe how he experienced going blind or what he thought about the matter.
He takes steps to counteract the disability. His mother moves to New York, becoming “his constant companion and help,” as a Raymond High School newspaper describes her in early 1941. His response to losing his sight is to “overcome the handicap, and not mention it,” one colleague says many years later.
“I just kept on going,” he tells a news reporter in 1948.
Behind Woolley’s determination are many people providing the accommodations he needs to just keep on going. His mother runs his home in New York while he learns how to live as a blind person. Gasser sets Woolley up in an independent lab, giving him space, facilities, and funding to pursue his research. While Woolley continues to work in the lab by feel and memory, he also relies on two assistants to whom he can entrust laboratory work that requires sight. They report their observations while Woolley plans experiments, makes deductions, and dictates publications. By the late 1940s, he also has three PhD chemists working under his supervision.
Other crucial accommodations come from McCarter and colleagues in Woolley’s lab. They spend untold hours taking turns reading scientific papers to him. Every biographical article about Woolley mentions McCarter reading to him. It’s the only personal detail these biographies reveal about her.
McCarter sacrifices her own research career to support Woolley. After completing her Guggenheim Fellowship and getting married in June 1945, she remains on leave from her professorship at the University of Wisconsin. By 1948 Woolley tells a news reporter that his wife still “shoots a guinea pig now and then” between her household duties, but her publication record had dried up. She never returns to an academic position, though she continues to attend scientific lectures in New York for decades.
By contrast, Woolley’s research thrives.
Respected Scientist and Mentor
After beginning his career studying growth factors and vitamins, Woolley turned his attention to the other side of the biochemical coin—antimetabolites, chemicals that inhibit growth and disrupt metabolism.
Metabolites are those molecules necessary to the processes of metabolism or those formed along the way. As biochemists cataloged these molecules, they also began to explore what became known as antimetabolites. Antimetabolites have chemical structures very similar to metabolites, but they block or disrupt the metabolic process. If metabolites and the chemical receptors they activate fit together like keys and locks, antimetabolites slide into the locks and jam them shut.
Antimetabolite research became a hot topic after the development of sulfanilamide, an antimetabolite-based drug, in 1935. As the first medications to cure bacterial infections, “sulfa” drugs offered hope to people like President Franklin Roosevelt’s son, who received sulfa treatments and was cured of a life-threatening strep infection in 1936.
Sulfanilamide worked by mimicking the chemical structure of an enzyme crucial to bacterial metabolism. Essentially the drug starved the invading bacteria.
Beginning in 1941 Woolley began to look for chemicals that blocked the action of vitamins by mimicking their chemical structure. In 1946 Woolley’s antimetabolite research uncovered the chemical factor in corn that blocks the action of niacin, leading to pellagra.

The antimetabolite work earned Woolley awards from various scientific organizations during the 1940s, including the American Society of Bacteriologists, the American Pharmaceutical Manufacturers Association, and the American Chemical Society. His 1952 book, A Study of Antimetabolites, received appreciative, even glowing reviews in the scientific press. “Here is a book that makes one proud to be a modern scientist,” gushed one reviewer.
Woolley used the award ceremonies as an opportunity to slyly show off to people who knew he was blind. Before a public lecture, Woolley would learn the location of the screens displaying his slides. During the talk he would punctuate each assertion by pointing confidently to its supporting data. He could write easily on a blackboard while he spoke. He lectured with such “sensitivity, intelligence and gifts of innocent showmanship,” a colleague said, that the audience often didn’t realize he was blind. He was proud that many of the people who knew him only from his public talks were unaware of his disability.
Woolley likely staged theatrical demonstrations to ensure colleagues would not consider him lessened by his blindness.
Bruce Merrifield met Woolley in 1949 while interviewing for a postdoctoral fellowship at the Rockefeller Institute. His future mentor was at a lab bench, preparing a specialized diet for his lab animals. Woolley held a huge 12-liter flask over his head and precisely poured the contents into a 10-inch Büchner funnel. He always did the mixing, grinding, and measuring himself, Merrifield remembered, so that “there would be no mistakes.”
Merrifield got the fellowship, and he and Woolley spent more than a decade working together.

“Woolley worked night and day in the laboratory, and when not doing experiments he was thinking about them,” Merrifield recalled. More than once, the cleaning staff were surprised to turn on the lights in the morning and find the chemist still working. (His home workshop didn’t even have lights.)
Though tireless in his own research, Woolley was a patient mentor, and that patience paid great dividends for science.
In May 1959 Merrifield had an idea for producing peptides one amino acid at a time in a way that could be automated. These chains of amino acids serve many functions in the body; some are neurotransmitters and hormones, while others are important to immune responses and metabolism. Building peptides to order would open great avenues for research.
It took Merrifield three years to develop the process and the machine to do it, during which time he produced no publishable results. Woolley let Merrifield persist. Two decades later, when a call from Stockholm reached Merrifield in his office, informing him he had won the 1984 Nobel Prize in chemistry, a picture of Woolley hung over his desk.
The Serotonin Hypothesis
The final act in Woolley’s research career began with LSD.
First synthesized in 1938, the molecule’s psychoactive properties were discovered in 1943. “Psychedelics excited many researchers” during the 1950s and early 1960s, writes historian Kim Hewitt, because they thought the drugs might help them better understand both mental illness and mystical experiences.
“There is no evidence that Woolley experimented with LSD by taking it,” Hewitt writes, “but it is an interesting coincidence that both he and Aldous Huxley were visually limited and able to make wide-ranging connections between aspects of psychedelic experiences and mental functioning.”

However, Woolley’s initial interest in LSD was as a potential heart medicine. During the late 1940s, researchers studying high blood pressure had isolated the hormone-like substance serotonin from blood and found it caused the walls of arteries to constrict. Recognizing the similar chemical structures of serotonin and LSD, Woolley wondered if LSD might function as an antimetabolite, blocking the action of serotonin. In 1952 and 1953 he and his lab colleague Elliott Shaw showed that LSD and other chemicals structurally similar to serotonin could prevent artery walls from constricting when serotonin was introduced.
Then in October 1953 Betty Twarog and Irving Page found serotonin in brain tissue, which raised the question of the chemical’s role in the nervous system and maybe even the mind.
Woolley knew from his extensive reading that LSD could produce strange mental effects similar to mental illnesses that at the time were labeled “schizophrenia.” Could it be that some mental illnesses were caused by metabolically induced serotonin deficiencies, similar to how diabetes was caused by a metabolically induced insulin deficiency? Woolley and Shaw wrote a paper laying out the theory and mailed it off to the prestigious medical journal The Lancet.
The medical community didn’t know what to make of the claims. The journal’s editors rejected the paper. It was not enough to simply suggest a biochemical basis for mental disease. To impress psychiatrists, Woolley would have to prove his point by actually curing mental illness.
Woolley and Shaw eventually published their paper, “A Biochemical and Pharmacological Suggestion About Certain Mental Disorders,” in the Proceedings of the National Academy of Sciences, a multidisciplinary journal more open to theorizing. They hoped the paper would spur psychiatrists to try serotonin treatments.
“Being only biochemists,” they wrote, “we are unable to do these experiments on patients and can only hope that this paper will stimulate those who are professionally qualified to undertake in man what we cannot pursue further in laboratory animals.”
In the meantime, Woolley dove ever deeper into medical psychiatry.
Woolley gathered evidence that might connect mental illnesses to chemical imbalances. He asked Janet and his colleagues to read to him from psychiatric and pharmacological journals. He traveled to conferences, including the Freudian gathering in Zurich in 1957. He also took experimental techniques developed in psychology and adapted them for testing the effect of neurochemicals.
Some of Woolley’s experiments focused on whether the behavior of laboratory animals could be used to assess their states of mind. He gave LSD to mice and watched what happened. After identifying a set of abnormal behaviors induced by the drug—because how can you know what feels psychotic to a mouse?—he then showed how those behaviors could be reversed or partially prevented by introducing serotonin as well as drugs known to affect the action of acetylcholine, a neurotransmitter.
Erasure
By 1962 Woolley feels ready to synthesize his evidence in a book, The Biochemical Bases of Psychoses, or The Serotonin Hypothesis About Mental Diseases. He frames the serotonin hypothesis as an alternative to “the Freudian concept of mental disease,” the idea that “certain baleful experiences of the earlier life of the sufferer” caused most mental disturbances. (Tell me about your mother. . . .)
The book begins playfully. Woolley teases the famous psychiatrists of Zurich and New York, jesting that the Freudian view is “apparently held widely among psychiatrists and informed laymen, especially in the great cities, although many sensible people have been reluctant to accept such a concept.”
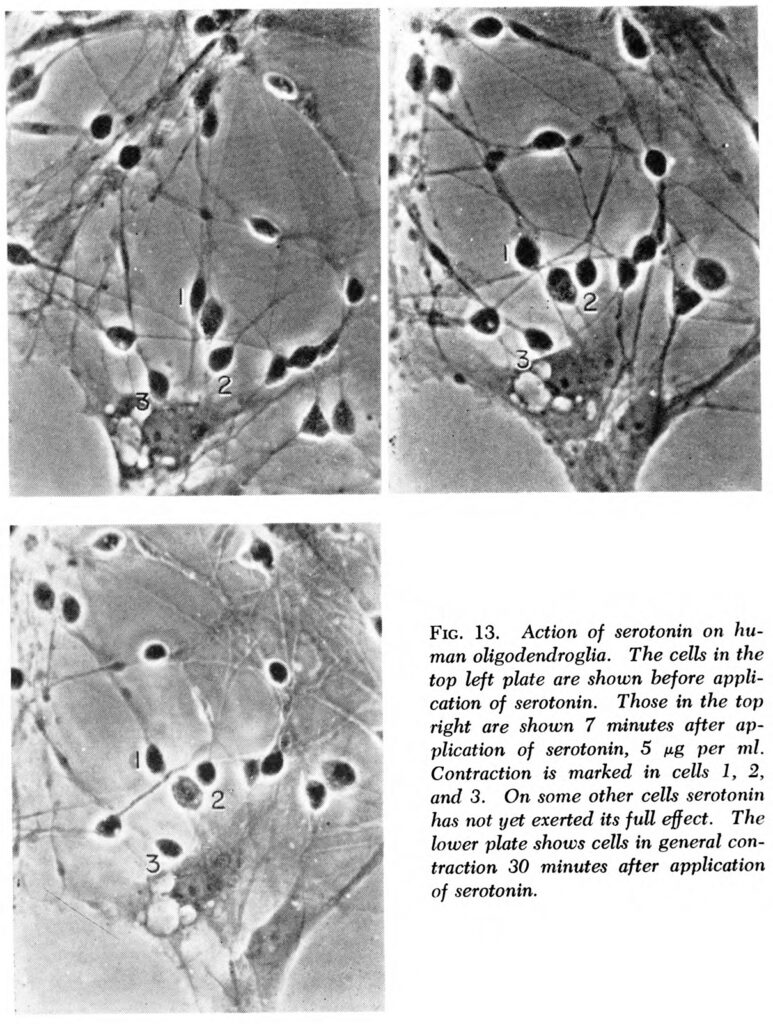
Then Woolley gets down to business. He explains his own path to the serotonin hypothesis. He notes that the dementia associated with pellagra never produced visible lesions in the brain, and promptly receded when patients got the right vitamin. He describes inducing psychotic behavior in rats with LSD and counteracting it with neurotransmitters. He follows with a litany of molecules found in human bodies and the ways these have been observed to affect smooth muscle, the nerves, and the brain. He says the understanding of mental illness in the 1950s is like medicine before germ theory, so researchers should pay attention to bodily symptoms, not the ever-shifting names of psychiatric diseases. While allowing that some kinds of illness may indeed be psychogenic (that is, arising from deranged thoughts), he argues that psychiatrists and medical researchers ought to first look for the biochemical origins of various symptoms of mental illnesses by using animal models and lab techniques. What he calls the serotonin hypothesis is not just about serotonin but also an argument that physical causes rooted in brain biochemistry lead to many illnesses of the mind.
By the time Woolley puts together these ideas, infirmities of the body are catching up to him. In winter 1965 he is hospitalized after a stroke. Still, he pushes on.
On a side trip before a pharmacology conference in Brazil, he seeks to satisfy a long-standing interest in archeology with a hike at Machu Picchu. Doctors have warned him that the strain on his body at such an elevation would be dangerous. On July 23, 1966, he and Janet are in Cuzco, 11,200 feet up the Andes, when he suffers a fatal heart attack.
Woolley died as he lived, confidently doing what he wanted “in the full knowledge that it was dangerous for him,” one of his colleagues eulogized.
Woolley’s sudden death, combined with the emergence of the war on drugs, erases his final act. LSD and other psychedelic drugs are outlawed after becoming popular with recreational users and associated with the counterculture of the mid-1960s. Research into them grinds to a halt. The role LSD has played in serious scientific research is buried. Woolley’s old friend Thomas Jukes won’t even use the term LSD in his tribute to Woolley in a 1974 issue of the Journal of Nutrition, instead calling it “an antimetabolite of serotonin.”

What becomes of Janet McCarter Woolley?
After Woolley’s death she continues to read widely in the literature and attend scientific lectures in New York. She explores writing a biography of her husband, corresponding with the Rockefeller Institute to find records and gather stories, but it never comes to fruition. She eventually moves to Salt Lake City, where she dies on Sunday, January 28, 1996, at age 89.
McCarter’s obituary in the Deseret News recognizes her as a pioneering woman in science, citing her PhD. It goes on to describe her as “a devoted marital companion” who “assumed a critical role as [Wayne’s] scientific partner” and “rightfully shared in his numerous honors and recognitions.”
Legacy
Today LSD and other hallucinogenic substances are enjoying a medical and scientific revival, in part a result of changing attitudes to drug use and criminalization, and in part the result of transformations in how we think about the connections between mind and body. (Historians have followed suit: Hewitt describes her work on Woolley as “rehabilitating LSD history.”)
During the first third of the 20th century, the brain and nerves were understood as primarily electrical in nature. The nervous system relayed information via electrical impulses from the periphery to the brain. Like a chief executive at the top of a sprawling business, the brain weighed this input and issued commands, sending electrical pulses back down through the nerves to the rest of the body.
Discoveries in biochemistry beginning in the 1920s began to disrupt this telegraphic image of nerve action. As chemists found ways to isolate and identify bioactive substances present in bodies at low concentrations, they found amino acids and peptide chains everywhere, doing who knows what. Scientists gradually uncovered the roles played by these molecules in amplifying and dampening signals that flew back and forth between brain and body with no single controlling flow. Today we call these molecules hormones and neurotransmitters.
By the end of the 20th century, the nervous system looked less like AT&T and more like cybernetic soup. Researchers who straddled seemingly disparate fields, such as psychoneuropharmacologist Candace Pert, were calling neurotransmitters “molecules of emotion” and discussing the role of these chemicals throughout the body in affecting the way we think and feel.
Dilworth Wayne Woolley played a key role in this evolution. While diabetes was destroying his body, his marvelously equipped mind was transforming the way scientists understood illness. If he ever owed science anything, he repaid the debt many times over.
But Woolley’s story is about more than his contributions to scientific understanding. His experiences remind us that accommodations for disabled people should not depend on acts of generosity or benevolence. It reminds us of the costs imposed on people with disabilities, their allies, and society as a whole, by systems not designed to accommodate their needs. The world could easily have lost Woolley’s genius without the support provided by the Rockefeller Institute’s directors, colleagues, assistants, and a wife who surrendered an award-winning career of her own. What might the world have gained from the lab of Janet Woolley had she been able to continue her own bacteriology research? We can only speculate.