Kabul’s central hospital was already filled with 600 of the city’s sickest cholera patients when Zinaida Plankina arrived in August 1960. The Afghan government had sought out the Soviet epidemiologist and her team of experts based on her earlier success in stemming a cholera outbreak in East Pakistan (present-day Bangladesh). Plankina’s tools of choice were bacteria-destroying viruses known as bacteriophages, and her group arrived with enough of them to treat every cholera patient in Afghanistan.
Shortly after landing, Plankina and her colleagues met with the lead doctors at the Aliabad General Hospital for what she thought would be a planning session on how to best administer the bacteriophages. Instead the Afghan doctors told her they would not be using them. The treatments would interfere, they said, with the American-supplied antibiotics already in use at the hospital. Plankina and her bacteriophages were unwelcome.
The epidemiologist unwittingly had found herself caught in a dispute that had less to do with science than politics. The struggle between those who supported antibiotics and those who relied on bacteriophages was a Cold War skirmish, with doctors and patients in Afghanistan caught between the warring cultural and political traditions of the United States and Soviet Union.
Afghanistan had a complicated relationship with both superpowers during the Cold War.
Britain’s departure from the Indian subcontinent in 1947 left a political void in the region. Afghanistan’s prime minister at the time, Shah Mahmud Khan, made clear his intention to align with the United States, seeing it as a natural ally and successor to Britain. But the United States had little interest in Afghanistan and instead pursued an alliance with Pakistan, a neighbor and political foe of Afghanistan. By the 1950s the U.S. government had established relations with Pakistan, which eroded American relations with Afghanistan.
The Soviets, on the other hand, shared a border with Afghanistan, giving them a vested interest in shoring up the political stability of their poorer neighbor. From 1955 to 1979, Soviet leaders sent the ailing, landlocked nation aid worth more than $9.3 billion today. The Soviets’ foreign aid was also a way to show the world that Soviet Communism was not only exportable but successful. If the Soviet project in Afghanistan succeeded, they would be one step closer to winning the Cold War’s zero-sum game.
But when Plankina arrived in 1960, Afghanistan’s political alliance with the Soviet Union had yet to be cemented. There was still a lingering desire to align with the United States among the country’s elites. Afghan doctors were among those who wanted U.S. support, including antibiotics, miracle drugs then experiencing a “golden age” of discovery.
Cholera is a highly contagious and often fatal diarrheal disease that is caused by the Vibrio cholerae bacterium. The disease had ravaged communities for centuries, but outbreaks remained small and isolated until the growth of international trade networks led to the first cholera pandemic in 1817, which began in India and spread as far as Russia. Thirty-eight years later British physician John Snow famously showed that cholera spread through contaminated water. Sanitation measures based on Snow’s discovery slowed transmission of the disease, but these improvements were of little help to those already sick.
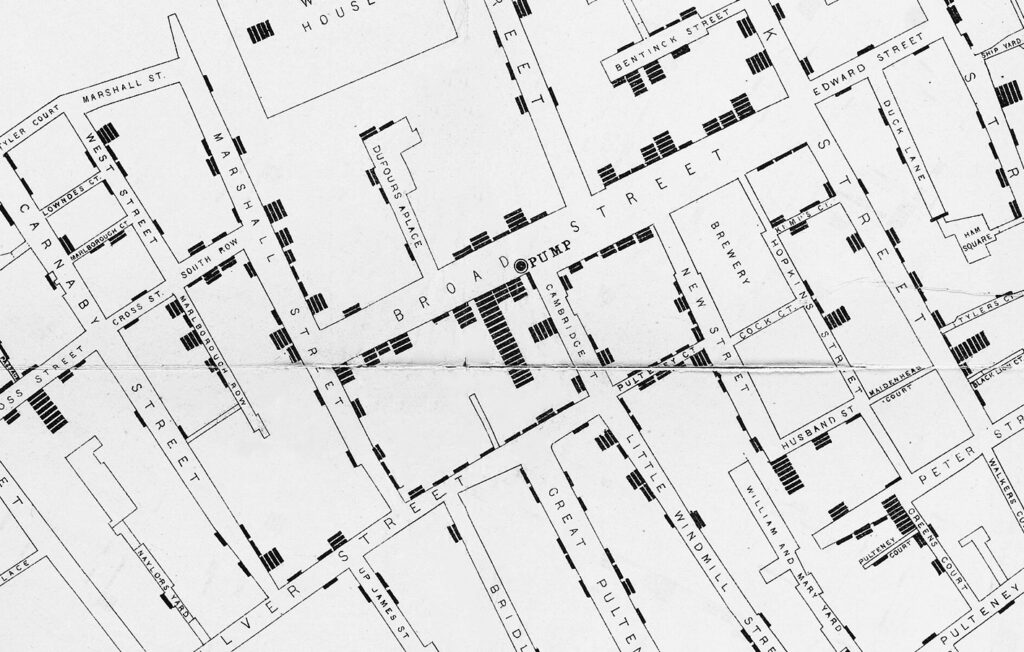
Detail of a map from London doctor John Snow’s investigation of an 1854 cholera outbreak, which he traced back to a contaminated drinking water pump.
In the next century powerful tools to fight bacterial diseases emerged.
Sulfa drugs arrived in the 1930s, followed by penicillin during World War II. These antibiotics could cure people, not just treat symptoms. By the time of the 1960 outbreak in Kabul, numerous antibiotics were being used to treat a variety of bacterial infections, including cholera. Partially as a result, the average life expectancy for Americans had increased by more than four years since World War II.
But antibiotics’ benefits were not shared equally. Going back to World War II, the U.S. government strategically limited access to the drugs. While antibiotic production boomed during the war, the U.S. military withheld them from their Soviet allies until the fighting was nearly over.
In response, Soviet scientists pursued therapies based on bacteriophages, continuing research that had been going for more than a decade. After the war the Soviet Union remained devoted to bacteriophage development, so much so that in 1953 it created regulations solidifying their status as the main treatment for bacterial infections and built research centers throughout the country to provide a steady supply of medicine.
But while the Soviets were early and eager adopters of bacteriophage therapies, they were not the first to isolate these remarkable viruses.
Soon after the start of World War I, British bacteriologist Frederick Twort realized that soldiers who were shot or otherwise sustained open wounds and then spent extended time in ponds or other bodies of water tended to fare better than those who fell on dry land. Intrigued by this phenomenon, he began to sample water from these sources and soon isolated a perplexing substance. Twort published his findings in The Lancet in 1915, describing the substance as made up of “ultra-microscopic viruses.” But Twort hedged his bets and suggested it could just as easily be “a minute bacterium” or an amoeba of some sort.

French bacteriologist Félix d’Hérelle, ca. 1905.
Twort’s failure to fully comprehend his findings was fortuitous for Félix d’Hérelle, a restless, adventure-seeking French microbiologist who set out to understand Twort’s discovery as well as the mechanism by which it healed wounded soldiers. Working at the Pasteur Institute in Paris, d’Hérelle soon determined that the mysterious agents were parasitic viruses, which he named bacteriophages (phage is Latin for “to eat”). In short order d’Hérelle published his findings on these bacteria eaters.
The world seemed poised to accept not only bacteriophages but a new approach to medicine, one that could cure the previously incurable. Bacteriophages became a part of the zeitgeist. Author Sinclair Lewis’s 1925 novel, Arrowsmith, about a doctor who uses bacteriophages to save people on a tropical island from a plague outbreak, won the Pulitzer Prize for Fiction the following year. D’Hérelle launched a short-lived bacteriophage laboratory in Paris and traveled the world—accepting awards supporting bacteriophage programs targeting dysentery, plague, and cholera—and held a professorship at Yale University for five years. By the 1930s bacteriophage processing plants were opening across the globe, including in the United States, France, and Brazil.
By that time d’Hérelle, like many intellectuals, had become disillusioned with political turmoil that had lingered in France since the end of World War I. The Soviet Union, with its message of unity and equality for all, seemed a better place for the abiding critic of capitalism, a place where he and other scientists could safely express their ideas. When a former colleague, Soviet bacteriologist George Eliava, offered d’Hérelle a job helping build a bacteriophage center at Eliava’s Institute of Bacteriology in Tbilisi, Georgia, the Frenchman jumped at the chance.

Georgian microbiologist George Eliava, undated.
Eliava’s brainchild was planned as a sprawling, 17-hectare, world-class facility. The plans were personally approved by Stalin, and included residences for both Eliava and d’Hérelle, laboratories, clinics, and even a vivarium, all with French motifs. D’Hérelle’s wife, Marie, spent a total of 12 months at the Institute in 1934 and 1935.
But toward the end of 1935, the political atmosphere shifted. Lavrentiy Beria—first secretary of the Georgian Communist Party, close Stalin ally, and childhood rival of Eliava—refused to provide more funds for the laboratory. Soon after, d’Hérelle and his wife boarded an Italian ship from Georgia’s port city of Batumi, under the pretense of needing to complete work at the Pasteur Institute. They never returned. In a harsh twist of fate, Eliava was murdered on Beria’s orders in 1937 during Stalin’s Great Purge.
The world seemed poised to accept not only bacteriophages but a new approach to medicine, one that could cure the previously incurable.
D’Hérelle continued to work on bacteriophages, but never gained the recognition he sought. He was nominated for a Nobel Prize several times but was never chosen. His scientific methods were repeatedly attacked, and he was placed under house arrest in Vichy France. He died in 1949, just as the golden age of antibiotics was beginning.
Despite these losses, Soviet bacteriophage research thrived. Several research centers opened across the Soviet Union, including Eliava’s lab, which was completed in the late 1930s. The laboratory in Tbilisi, now named the Eliava Institute, is still producing bacteriophages for the citizens of Georgia and surrounding countries.
An effective bacteriophage treatment for cholera remained elusive until 1954, when Soviet researcher Aleksandr Grigorevich Nikonov at the Rostov-on-Don Anti-Plague Research Institute finally succeeded. Nikonov’s success required meticulous trial-and-error experiments on cholera-infected guinea pigs as well as the development of new growth mediums for bacteriophages, which included such ingredients as “bile, the contents of the small intestine and fragments of the small intestine in Tyrode’s solution.”
In 1958, when an outbreak of cholera struck a city in a remote part of East Pakistan, the Soviets seized the opportunity to show the world that their bacteriophages were at the very least as potent as their rival’s antibiotics. In a matter of weeks the Soviet contingent had treated everyone in the city; not a single person showed signs of recurrence.
While armed with a proven cholera treatment, the outbreak Plankina and her colleagues encountered in 1960 was particularly daunting. Cholera was a common problem in Afghanistan—the country had experienced several outbreaks in the previous 40 years—but modern transportation had expanded the disease’s reach and ramped up the pace of its spread. Plankina also recognized that some traditions were contributing to the spread. Irrigation ditches were used as a place to wash corpses and a source of water for rinsing the mouth. The Kabul outbreak was later traced back to a woman who drank from an irrigation ditch, where, further upstream, mourners had washed the linens of a cholera patient from Jalalabad.

An Afghan vaccine research lab, ca. 1960, from Afghanistan: Ancient Land with Modern Ways, 1961.
Doctors at Aliabad General Hospital initially tried to isolate the sick, which proved ineffective; it was simply too difficult to isolate people quickly enough to blunt the spread. And the American antibiotic they were using, oxytetracycline, was only about 50% effective.
Faced with a growing number of cases, the hospital’s doctors began testing and quarantining anyone who had come into contact with a positive case. But they were too late. The outbreak surged and overwhelmed the staff’s capacity to test patients. By the time Plankina arrived in August 1960 all of Aliabad General’s 600 beds were full, the cholera patients crowded close together but without the proper isolation measures that would prevent further spread within the hospital.
On October 2 a patient on the neurological ward of another hospital died of a diarrheal disease. A hospital worker who cleaned the body and a cook who fed the patient also became ill. By October 6, 12 patients in different parts of the hospital came down with the same symptoms, which were similar to those of cholera. Because these patients were scattered throughout the hospital and not contained within the cholera ward, the overwhelmed Afghan doctors failed to recognize the cases as cholera.
When Plankina heard about the mysterious bout of gastroenteritis, she seized the opportunity to test those patients for cholera. After the patients, including two who were comatose, tested positive, Plankina secretly treated them with bacteriophages. By the next day these patients had improved so rapidly that the Afghan doctors abandoned their antibiotics and switched to Plankina’s bacteriophage injections. Locals reportedly dubbed the treatment “holy water.”
Between October and December 1960 Plankina and her colleagues inoculated 1,600 hospital employees with bacteriophages. Through that winter the Afghan minister of public health corralled foreign aid from the World Health Organization (WHO) and persuaded scientific teams from France and Czechoslovakia to come help Plankina and her colleagues inoculate people in Kabul and its environs. The international team used more than 550 liters of the cholera bacteriophage to treat more than 27,000 people, some living in villages that are among the world’s highest and most precarious to reach. Not one person treated with bacteriophages became ill with cholera in three years of follow-up surveillance.

A remote Afghan village in the Hindu Kush mountains, ca. 1969.
Despite the remarkable success of this medical mission, just one English-language article on Plankina’s work exists, published by the WHO. She and her colleagues returned to the Soviet Union much as they had come, with little fanfare. She was still Soviet, after all, and her science was still seen as other by the Afghans and members of the WHO delegation.
Why did no enterprising or ambitious American researcher adopt such a compelling treatment? It’s likely the English-speaking world never knew of her efforts. The teams that helped distribute the bacteriophages were French, who had a bacteriophage connection through d’Hérelle, and Czech, who were then in the Soviet sphere of influence.
When it comes to U.S. bacteriophage research, there has been little growth in the 60 years since Plankina set out on her Afghan mission. But that might change as doctors face an increasingly alarming but long-recognized problem.
Soon after antibiotics were discovered, signs of bacterial resistance began to emerge. Scientists, such as future Nobel laureate Selman Waksman, realized as early as 1945 that bacteria were becoming resistant to penicillin. Two years later Waksman’s bacteria showed resistance to streptomycin. Newer, so-called broad-spectrum antibiotics would be needed to overcome the growing resistance, but bacteria showed signs of resistance to these newer drugs almost immediately. Waksman even saw signs of resistance to streptomycin while conducting efficacy studies on experimental guinea pigs.
Belgian microbiologist Maurice Welsch became convinced that resistance was unavoidable and in 1952 told the WHO as much. Pharmaceutical companies responded by introducing fixed-dose, combination antibiotics to overcome rising bacterial resistance. These drugs combined two, sometimes three, antibiotics in one pill, but were banned in the 1960s due to increasing bacterial resistance.
Advertisement for the broad-spectrum antibiotic oxytetracycline, ca. 1979.
Oxytetracycline, the antibiotic used in Kabul in 1960, belonged to the broad-spectrum family. This pale-yellow drug was developed in 1952 by Pfizer scientists, the first antibiotic to be produced entirely by a pharmaceutical company. The company branded it Terramycin because it was found in soil samples and proved successful in treating a variety of bacterial infections.
Despite growing antibiotic resistance, bacteriophage treatments never took off in the antibiotic-dominated West. Historians have speculated that because antibiotics held so much promise in the early years of their development, scientists struggled to understand the inevitability of resistance for all antibiotics. Certainly, these researchers had a reason to be optimistic. Scientists were creating antibiotics at a rapid rate, and when resistance was recognized, new antibiotics, such as vancomycin, were made, often in the belief that no bacteria would ever develop resistance to them.
This boundless support for antibiotics and their promise was buttressed by the Cold War and its effects on American and Soviet science. The conflict influenced scientific fields on both sides of the political divide. For example, Soviet scientists promoted their own form of genetics, which relied heavily on theories championed by Trofim Lysenko, primarily because it was not Western.
Why did no enterprising or ambitious American researcher adopt such a compelling treatment?
Americans felt similarly disdainful of Soviet medicine. In a CIA report from 1951, analysts examining the state of Soviet medicine concluded that it was of the “simplest old-fashioned type.” The Cold War’s influence on this kind of analysis is apparent.
While there’s no clear evidence the CIA buried knowledge of the Soviets’ success with bacteriophages, American scientists’ unwavering pursuit of antibiotics and their abandonment of bacteriophages aligned with similar Cold War policies, which favored the adoption of homegrown science. (The Soviets, it’s worth noting, did develop their own antibiotic, called gramicidin-S, although a lack of resources and other factors prevented them from developing other antibiotics.)
A Cold War hangover may be partially to blame for Americans’ enduring reluctance to pursue phage therapies, but money might be a more important factor: bacteriophages, as naturally occurring organisms, cannot be patented.
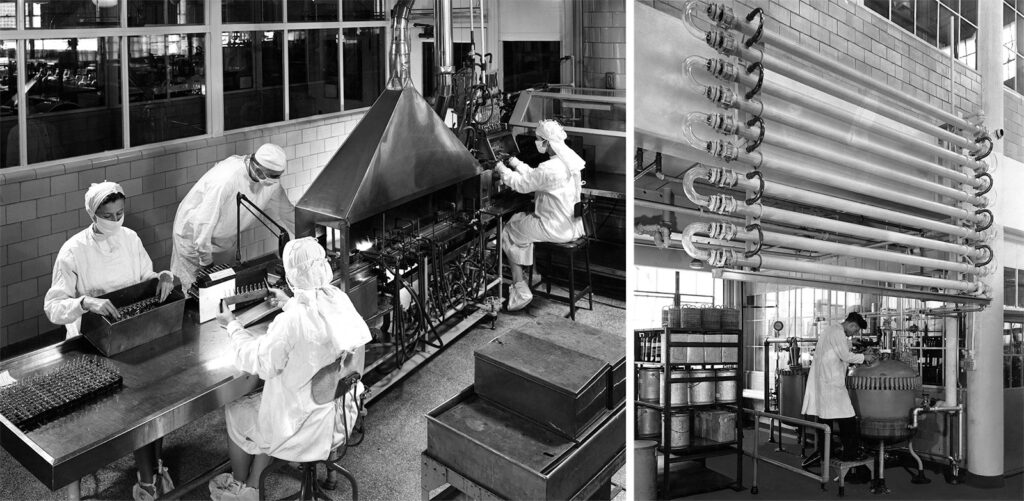
Antibiotic production at the Parke, Davis Research Laboratory in Detroit, Michigan, ca. 1940s.
Phage therapies are effectively banned in the United States and in most other Western countries. However, rising antibiotic resistance has at least some Americans taking another look at the viruses’ potential.
A notable example is a team of doctors at the University of California, San Diego, who in 2016 successfully lobbied the FDA for emergency use authorization for bacteriophages to treat Tom Patterson, a professor of psychiatry at the school, after he became infected with Acinetobacter baumannii—a life-threatening, multi-drug-resistant bacteria—during a trip to Egypt in late 2015.
The treatment’s remarkable effect mirrored those seen with Plankina’s patients more than 50 years earlier. After receiving the bacteriophages intravenously, Patterson came out of his monthslong coma within three days. He returned to work, fully recovered, shortly thereafter. This success, along with a few others, prompted several researchers to launch the school’s Center for Innovative Phage Applications and Therapeutics in 2018 to combat antibiotic-resistant diseases.
The San Diego lab is an outlier. Many U.S. researchers doubt the efficacy of bacteriophages, and there is some evidence supporting such skepticism. Recent research, for example, has found that the body can quickly shed bacteriophages. In such cases bacteriophages do not spend enough time in the body to destroy bacteria, rendering the treatments useless.
Yet Georgian scientists have successfully treated patients with bacteriophages since the 1930s. Any suggestion that they are ineffective runs counter to decades of their own research and clinical experience. Today patients can buy ready-made bacteriophages for specific bacteria, such as staphylococci, and can receive a tailor-made bacteriophage cocktail within three days for other, more complex bacterial infections. The phage therapies made by the Eliava Institute’s doctors today have not changed much since the 1930s. For them the treatments simply work.

Bacteriophage researchers Nina Chanishvili (left) and Ketino Porchidze at the Eliava Institute in Tbilisi, Georgia, June 2005.
This was the case for Alfred Gertler, a Canadian who in 2001 became the first Westerner treated with bacteriophages in Georgia. A year earlier he had read an article in the New York Times Magazine about bacteriophages. The article highlighted their absence in the West, despite the Georgian scientists’ success in using them to treat bacterial infections, such as the Staphylococcus bacteria that had long ravaged Gertler’s foot and continued to do so even after nearly four years of antibiotic treatments.
Gertler knew he would soon lose his foot and viewed phage therapy as a last-ditch chance to avoid amputation. He soon discovered bacteriophages were not an approved treatment in the West. So in early 2001 he spent nearly all his savings to fly to Tbilisi and begin treatment.
After two weeks of treatments at the Eliava Institute, Gertler was cured and walked out of the hospital. Despite this success just a handful of Westerners have been treated in Georgia since.