When Linus Pauling celebrated his first Nobel Prize, for chemistry in 1954, the bonds between the scientist and Caltech seemed unbreakable. The university marked the win with a dinner for 350 guests, including a faculty musical, The Road to Stockholm. “Dr. Pauling’s never wrong,” the singers insisted. “And his double bonds are strong.”
But Pauling’s colleagues also issued a warning that night:
Every chemist needs his patrons, filthy lucre can’t be spurned
But don’t forget that Galileo very nearly burned
So make those associates smile.
It was a warning the famous chemist failed to heed. Instead, when Pauling won his second Nobel in 1963—a peace prize for his work toward nuclear disarmament and a testing ban—the reaction on campus was noticeably muted.
Pauling’s years of peace advocacy had alienated many of Caltech’s associates and benefactors. Before leaving for Stockholm to collect his prize, Pauling had only a quiet coffee with members of the biology department. By then he had cleared out his desk and resigned from the school he had called home for 40 years, pushed out by conservative trustees who pressured the university to cut his salary and remove him as chemistry department chair.
The exodus from Caltech was a massive blow to the revered scientist, says Chris Petersen, an archivist at Oregon State University. Stripped of his scientific community, funding, and facilities, Pauling’s career foundered. “He was fighting an uphill battle for the rest of his life,” says Petersen.
This battle ultimately led Pauling to start a maverick crusade promoting the curative powers of vitamin C. It was a campaign that would define the last few decades of his life and, for many, tarnish his scientific reputation.
“The way he approached his vitamin C work was a curious (and sad) mixture of the same boldness that had served him so well in the past, but now with a messianic strain that would probably have proven fatal to much of his own earlier work,” Science columnist and pharmaceutical chemist Derek Lowe wrote in 2014. “Self-confidence is absolutely necessary for a great scientist, but too much of it is toxic.”
Nearly 60 years after he began his crusade, Pauling’s legacy is a mixture of brilliant discoveries and misguided yet intensely fought battles. But was Pauling’s advocacy of vitamin C simply a matter of desperation and hardheaded confidence?
As a teenager, Linus Pauling taught himself chemistry in the basement of his Portland, Oregon, home, building a laboratory with materials scavenged from an abandoned iron smelter. Though he didn’t finish the coursework to earn a high school diploma, at age 16 he was accepted into Oregon Agricultural College (now Oregon State University), where he proved himself a chemistry wunderkind. When he ran short of money, the college allowed him to teach chemistry courses. In one, he met his future wife, Ava Helen Miller.
The couple married and moved to Pasadena, where at Caltech Pauling researched crystal structures and earned a doctorate in 1925. A few years later Caltech hired the young chemist, and there Pauling paired his experience with X-ray crystallography with an understanding of quantum mechanics to conduct groundbreaking research on the arrangement of atoms in a molecule. He explained his orbital hybridization, or valence bond theory, in 1939 in his seminal textbook, The Nature of the Chemical Bond and the Structure of Molecules and Crystals.
“One of his superpowers was that he could synthesize across disciplines,” says Petersen. “Pauling hoovered up information and could recall it years later.”
By 1941 Pauling was 40, married with four children, tenured at Caltech, and decorated with prestigious awards. To support the U.S. war effort, he joined the National Defense Research Committee and began to research rocket propellants and oxygen meters for use in submarines and airplanes.

But that year he started to feel sick. His throat became so sore and swollen he couldn’t button his shirts. He was diagnosed with a potentially deadly form of kidney disease called glomerulonephritis. To treat the disease, he followed the advice of Thomas Addis, a nephrologist at Stanford University who prescribed a low-sodium, low-protein diet intended to give the kidneys time to rest and recuperate. Pauling became a vegetarian, and Ava Helen kept careful logs of what and how much he consumed. The treatment worked. Petersen suggests this was an eye-opening moment for Pauling, who saw firsthand how particular nutrients could affect his health.
But Addis did more than change Pauling’s diet. Addis was a passionate anti-fascist who chaired an organization that advocated for the release of political prisoners in Franco-controlled Spain and led the San Francisco chapter of the Physicians Forum, which campaigned for universal healthcare. Addis encouraged Pauling to become more involved in politics. With his increased success and prominence came an imperative to advocate for peace.
Others Pauling respected voiced similar sentiments. Under the influence of his wife and pressure from scientists such as Albert Einstein, Pauling became a trustee of the anti-proliferation group the Emergency Committee of Atomic Scientists and a more vocal figure. In 1947 he wrote himself a pledge to “include mention of world peace in every lecture and address that I give.” He urged others to do the same with the messianic intensity that would become a hallmark.
“The problems that the world faces are great, serious, and difficult,” he wrote in Chemical and Engineering News. He urged scientists, armed with a unique understanding of the dangers of nuclear weapons, to take a stand. “Chemists and other scientists have a social obligation which is greater than that of the ordinary citizen,” he wrote.

In a country gripped by McCarthyist paranoia, Pauling’s calls for nuclear test bans and disarmament got him branded a Communist and traitor. His home phone rang with death threats. In 1952 the U.S. State Department refused to renew his passport, stating that doing so “would not be in the best interests of the United States.” The department relented only after his Nobel Prize in Chemistry was announced in 1954.
Despite it all, Pauling carried on with his advocacy. He published scientific papers estimating the effects of nuclear weapons testing on future generations. He testified before Congress. He picketed the White House. He rallied thousands of scientists to the nonproliferation cause.
By the time he won the Nobel Peace Prize in 1963, he was a scientific celebrity on par with Albert Einstein. He was also considered toxic by most research institutions, which relied on government grants to fund their labs.
Amid all this political and personal turmoil, Pauling was digging into a new field of research. He would ultimately call it orthomolecular medicine, combining the Greek “ortho,” meaning straight, correct, or right, with “molecule.” With the right titration of molecules, he believed, doctors could cure a range of health problems, from the common cold to schizophrenia, autism, cardiovascular disease, and cancer.
“Man is simply a collection of molecules,” he remarked in 1956. Therefore, humans “can be understood in terms of molecules.”
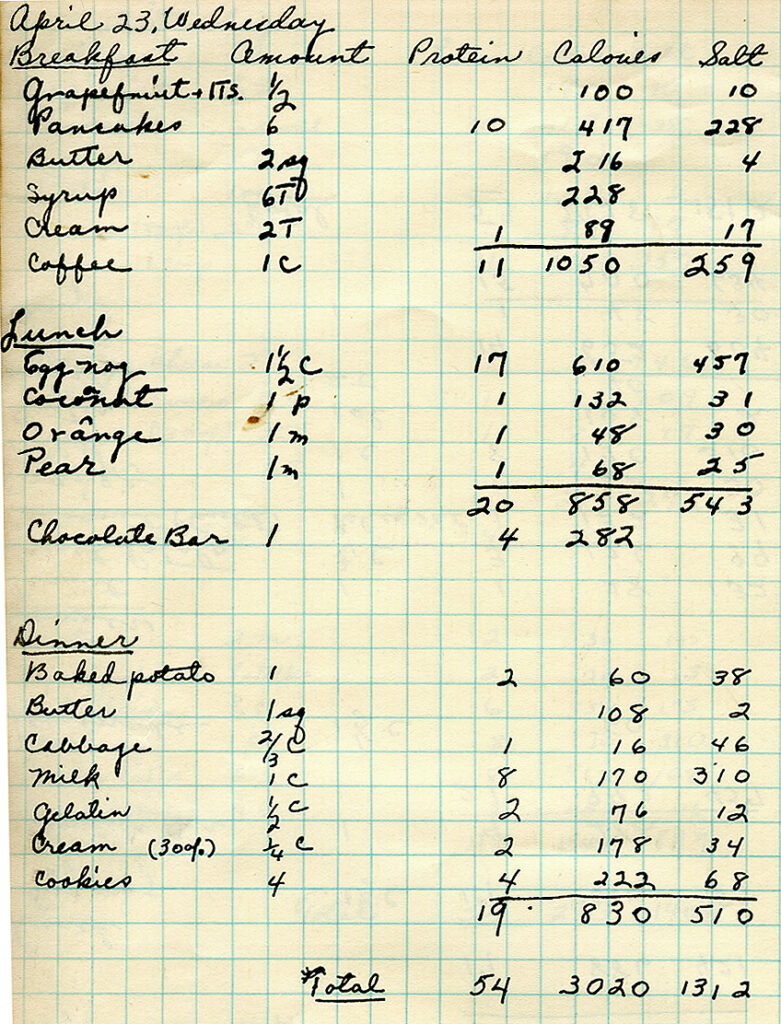
Pauling had already had remarkable success in molecular biology, employing the techniques he had used to visualize the structures of chemical bonds to describe the structures of proteins. In 1940 he published an influential paper on how antibodies form. His theory was off, but his work inspired experimentation by others that ultimately led to our understanding of how the immune system works.
In 1945 he heard a scientist describe the characteristic half-moon shape of red blood cells in patients with sickle cell anemia. Pauling hypothesized this shape was caused by a problem with oxygen-carrying hemoglobin molecules. In 1949 he co-authored “Sickle Cell Anemia, a Molecular Disease.” The paper not only established that the disease could be traced back to molecules, it also demonstrated that genes determine how proteins form.
That early success in linking molecular abnormalities to disease got Pauling curious about ways he could use chemistry to improve human health. In that same 1956 public address, he said, “I believe that chemistry can be applied effectively to medical problems, and that through this application we may look forward to significant progress in the field of medicine as it is transformed from its present empirical form into the science of molecular medicine.”
In his last years at Caltech, Pauling began to research the evolutionary causes of molecular disease, arguing that the amino acid mutation that causes sickle cell anemia could be an intermediary step in evolution. After being forced out of the university, Pauling began to cast around for new problems to research.
“Fate threw him a curveball,” says Petersen, in the form of a chance encounter at a talk he gave in 1966.
During the lecture Pauling, now age 65, expressed a wish to live long enough to see realized all the scientific advancements on the horizon. Irwin Stone, a biochemist and engineer sitting in the audience that night, wrote to Pauling with information on a “high-level ascorbic acid regimen” that he suggested could help Pauling “possibly tack on a few extra decades.” Pauling was intrigued. He and his wife started taking vitamin C supplements. Over the next few years, they steadily increased their dosages and observed that Pauling was no longer dogged by the frequent colds that tormented him for much of his adult life.

Pauling took a short-term position at the University of California, San Diego, then moved to Stanford. In these posts, he began to apply his previous theories about sickle cell anemia to his interest in vitamin C. He developed an evolutionary explanation for the need to use vitamin C supplements using theories about a similar molecule called thiamine, which some animals lost the ability to synthesize.
Researchers had hypothesized that animals lost this ability because their food supply was rich in thiamine. Pauling argued that the same reason must explain why only a few animals—including a type of Indian fruit bat, guinea pigs, and humans—had lost their ability to create vitamin C. That is, for a sustained period of human evolution, humans were consuming so much vitamin C that the loss of synthesis exerted no evolutionary pressure. What’s more, according to Pauling’s calculations, the amount of vitamin C modern humans were eating was only the bare minimum needed to prevent scurvy but not enough to realize the full health benefits of vitamin C.
Here too Addis’s influence on Pauling is evident. “I now realize that Addis’s regimen was completely orthomolecular,” Pauling wrote in his biography of the doctor. “I received no drugs. My treatment involved only the regulation of the intake of substances normally present in the human body: increased intake of water, vitamins, and minerals and decreased intake of protein and, for a time, salt, combined with some rest in bed.”
But Pauling had conducted none of the studies his vitamin C theories were built on. Human drug trials are large and expensive to run. So instead, Pauling’s claims were based on the review of existing literature and the experiments of others. This invited problems, such as flawed data. The trials Pauling relied on had inconsistencies between study groups that made the placebo and the vitamin C groups nearly impossible to compare.
Despite his brilliance, Pauling was ill-equipped to catch these errors.
Pauling biographer Ted Goertzel points out that nutrition research isn’t quite like chemistry. There are many more variables to consider in a human trial than with isolated molecules in a lab. What is the dosage of the supplement? When and how is it administered? The study participants themselves add complications: their age, health, or the strength of their immune systems can all differ. Their lives and activities outside the lab can affect the results too.
“His vast knowledge was specialized,” says Goertzel. “He had tremendous knowledge on molecular structure and chemistry. But that doesn’t mean you have a tremendous knowledge about nutrition and vitamins and health.”
And Pauling’s interpretations were questionable. He emphasized the positive effects he saw while explaining away negative or weak results as flaws in study design. Pauling’s vitamin C research was full of cherry-picked data that he wove into a narrative that suited his hypothesis, a process Goertzel likens to the logic of conspiracy theories.
“You get selective evidence, and you throw something out and insist that other people disprove it, and if they can’t disprove it, it might be true,” he says.
Pauling’s approach to vitamin C had none of the rigor or peer review that characterizes good science. When other scientists criticized his articles in peer-reviewed journals, Pauling took his case straight to the public. In 1970 he published Vitamin C and the Common Cold. Written for general audience, the book extolled the virtues of megadoses of ascorbic acid using little more than anecdotal evidence.

Between his two Nobel Prizes, his peace advocacy, and his sickle cell research, Pauling had become an outright celebrity. When he got stuck on a cliff near his home in Monterey, it made national news. That fame powered the vitamin C book to mainstream success despite protest from the scientific community. Doctors and researchers argued that taking vitamin C in these quantities was a waste of money. Some researchers even accused Pauling of working as a shill for supplement companies.
Pauling dug in his heels. Like any scientist pushing their field forward, Pauling had proposed plenty of ideas over the years that were later proved false. He famously hypothesized that DNA had a three-strand structure rather than a double helix. But once he saw Rosalind Franklin’s images, he conceded he was wrong.
As he aged, however, Pauling’s always healthy ego betrayed him; he became increasingly intractable. During an argument over the discovery of structures in alloys called quasicrystals, Pauling purportedly announced that “there are no quasicrystals, just quasi-scientists,” referring to Dan Shechtman, the scientist who had made the discovery. (Shechtman won the Nobel Prize in Chemistry in 2011 for his work.)
In 1973, Stanford convinced Pauling to retire. The restless chemist, now age 72, instead set out to create his own independent lab, eventually named the Linus Pauling Institute. But the institute struggled to raise money. Pauling spent more time as an administrator and fundraiser than as a working scientist.
“He couldn’t get any grants because he was so controversial,” says Petersen.
Pauling also had alienated many in the scientific community. Not everyone agreed with his outspoken views on politics. And not everyone enjoyed working with him. Over the years, he had earned a reputation for taking credit for the work of many of his younger colleagues and students.
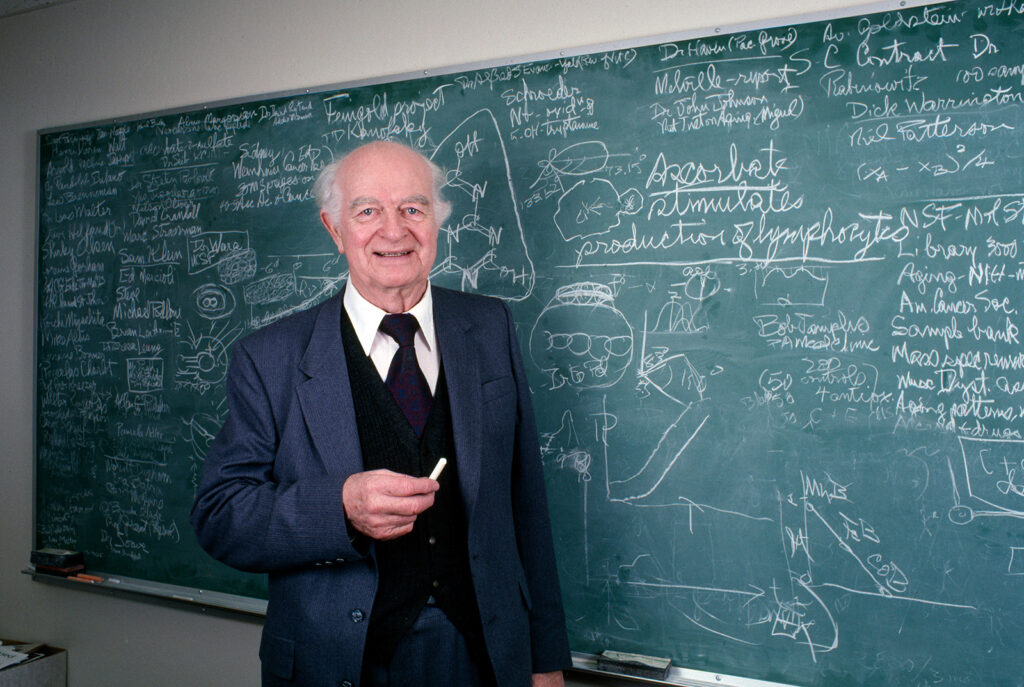
“A lot of the young scientists who worked with him felt that he would take credit for joint publications because he suggested the idea,” says Goertzel. Instead of being listed as first author, those younger scientists would be relegated to a long list of coauthors. “They called it being lost in the et als,” says Goertzel.
Pauling did begin to coauthor some papers on vitamin C, but he wasn’t conducting the research. Instead, he helped draft the manuscripts. The trials were overseen by a friend, Scottish physician Ewan Cameron. They examined how intravenous vitamin C might affect the lifespan of patients with terminal cancer.

The methods used in these studies were deeply flawed. The patients had different kinds of cancers, and their cases couldn’t be meaningfully compared. The trials weren’t blinded, and there was no control group to test whether the results could be attributed to placebo effect. The work was met with derision from the medical community. One scientist described the trials as “ill-defined and unreproducible,” adding that vagaries in the paper “would make it impossible for any other workers in the field to do a comparable study.”
Yet again, Pauling sidestepped these critiques and instead published Cancer and Vitamin C with Cameron in the trade press in 1979. In the book, they argued that vitamin C helps build collagen that strengthens cells against cancer while also helping to create an enzyme that can stop the breakdown of healthy tissue.
Pauling’s promotion of the book successfully goaded researchers at the Mayo Clinic into performing two trials of vitamin C effectiveness in treating cancer, in 1978 and 1984. But the investigators’ final report was decisively against Pauling:
Pauling was enraged. The Mayo trials had used oral vitamin C, while his study had used intravenous administration. They could not be compared. But the mainstream medical community had seen enough and largely moved on. Pauling continued the vitamin C crusade for the next eight years, using his own life as anecdotal evidence for vitamin C’s curative efficacy. Although doctors were unconvinced, the public embraced vitamin C and Pauling.
In 1993, during a lecture at the University of California, Berkeley, Pauling announced to the crowded auditorium that he had prostate cancer but suffered none of the usual side effects from chemotherapy because of the whopping 18 grams of vitamin C supplements he consumed daily—equivalent to eating about 360 oranges. A year later, he died.
Public interest in vitamin C far outlived Pauling. His crusading stoked a wave of consumption of over-the-counter supplements and fueled an industry that today is estimated to be worth more than $177 billion. Despite numerous studies that show vitamin C cannot prevent the common cold and does little to improve symptoms, products such as Emergen-C remain hugely popular. These products aren’t harmful, but they also don’t do anything.
“You’re just going to pee it out,” says Aimee Bernard, an immunologist at the University of Colorado. “It’s very expensive urine.”
That’s not a problem when you’re treating a cold. But proponents of alternative medicines point to Pauling’s vitamin C work as proof that supplements are important and undervalued treatments for much more serious conditions. When people take vitamin A instead of the measles vaccine, approaches that favor anecdote over evidence can be deadly.
Pauling’s flawed methods and combative style also may have delayed the realization of a dream he had so wanted to see.

Channing Paller, an oncologist and researcher at Johns Hopkins University, describes Pauling’s effect on vitamin C research as “unspoken and substantial.” The drama surrounding his fights with other scientists and the finality of the Mayo Clinic’s findings discouraged others from pursuing the idea. “It delayed the study for probably 15 years,” she says. “It really slowed the research because Mayo had said it was wrong.”
But in the mid-2000s Mark Levine, a researcher at the National Institutes of Health, began to test Pauling’s theory that IV vitamin C is absorbed differently than pills. His work showed that intravenous delivery allowed the body to build up much higher concentrations of vitamin C in its tissues. Those higher volumes of absorption could help deliver anti-cancer drugs and promote oxidative stress that can damage cancer cells.
Over the years, Levine and other researchers have built a promising body of evidence that suggests large doses of IV vitamin C—also called ascorbate—alongside chemotherapy, can have significant results both in limiting tumor growth and curbing the side effects of the chemo, though how it does this is still in question. It may be that vitamin C helps to reduce inflammation or that it increases production of neurotransmitters, such as dopamine and serotonin. Or it may be that while vitamin C acts as a pro-oxidant in tumors, it may have some antioxidant properties too.
“Should the oncology research community invest in the necessary randomized trials to test whether there is benefit of IV ascorbate? We firmly recommend yes,” Levine and his colleagues wrote in a 2018 Cancer Cell paper. “We strongly encourage the field to explore ascorbate’s potential, but also send a cautionary note that new studies need to be conducted properly. Let us not shoot down the re-rising ascorbate treatment phoenix.”
Even so, ascorbic acid is far from the miracle cure Pauling believed it to be. “Drug discovery is nuanced,” says Paller. “Chemotherapies work for some cancers but not others.”
In one study of 34 patients conducted by the University of Iowa and published in November 2024, researchers found that incorporating vitamin C into treatment plans nearly doubled life expectancy for patients with late-stage pancreatic cancer, extending median time of survival from 8.3 months to 16 months. However, Paller suspended a different phase II trial on vitamin C and prostate cancer after she and her collaborators found the drug was having no effect.
In this sense, making vitamin C into an effective treatment is the same as with any other drug. There is no magic cure-all. There will be no eureka moment. Instead, there will be years of testing and research, adding data point to data point until a clearer picture emerges.




